Omnipod® 5

Omnipod 5 Automated Insulin Delivery System is the first wearable, tubeless, hybrid closed loop system that integrates with the Dexcom G6, Dexcom G7 and FreeStyle Libre 2 Plus Sensors, for people with type 1 diabetes aged 2 years and older requiring insulin.
Three Simple Parts
Controller + Pod + Sensor
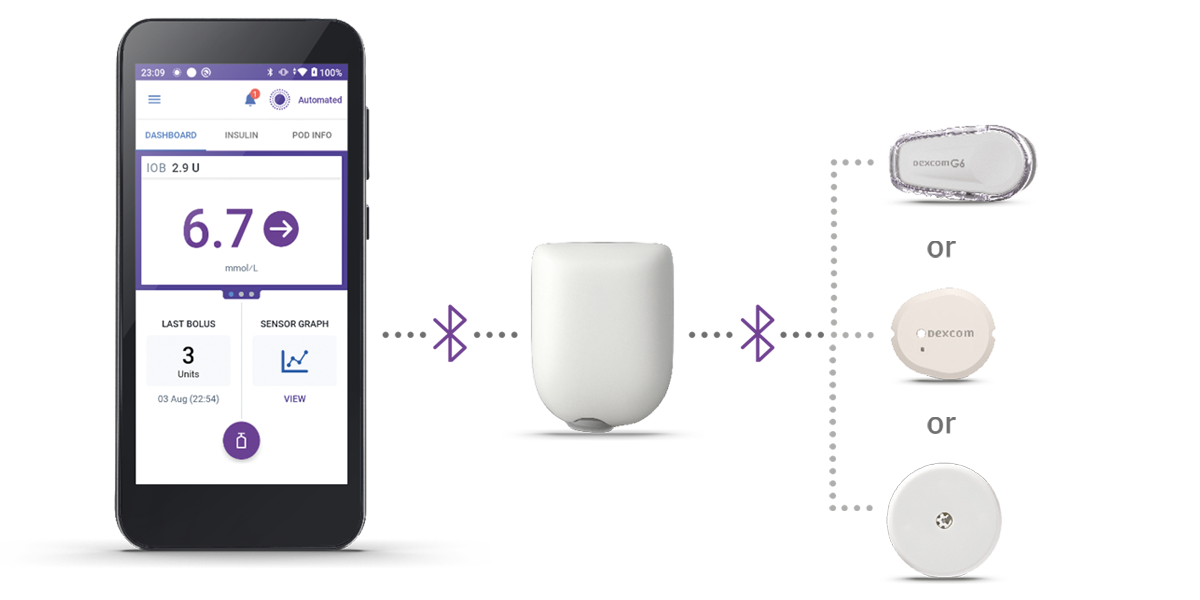

Omnipod 5 Controller
The Omnipod 5 Controller allows you to monitor and control the Pod using Bluetooth® wireless technology.

Pod
Tubeless, wearable, and waterproof,** the Pod, with built-in SmartAdjust™ technology, sits right on your patient’s body and automatically§ adjusts insulin delivery for up to 3 days or 72 hours.
§ Automated Mode requires compatible CGM Sensor
**The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Omnipod 5 Controller is not waterproof. Please consult Sensor manufacturer user guide for Sensor waterproof rating.


Sensor
Continuously sends glucose values to the Pod, so your patients can get real-time data without the finger pricks†.
†Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.


SmartAdjust™ Technology
Predicts
Glucose 60 minutes into the future
Adjusts
Insulin delivery using the selected Target Glucose
Delivers
Insulin doses every 5 minutes (as needed)
Always adjusting, so you don’t have to.
Omnipod® 5 with SmartAdjust™ technology automatically adjusts to your patients’ personal needs by increasing, decreasing, or pausing insulin delivery every five minutes – which may help protect against highs and lows.1,2 Watch this video to learn more.
Improved Clinical Outcomes
Omnipod 5 improved glycaemic control in adults, adolescents and children with T1D in pivotal studies. 1,2
76%
time in range (TIR) at a target of 6.1 mmol/L (110 mg/dL) in adults and adolescents (14–70 years) and 68% overall TIR in children (2- 13.9 years)1,2
HbA1c
was significantly reduced in very young children (2.0–5.9 years), children (6–13.9 years), adults and adolescents (14–70 years) by 0.5%, 0.7% and 0.4% (4.2, 6, and 7.8 mmol/mol) respectively1,2
33%
reduced time in hyperglycaemia in children and 24% in adults and adolescents1
60%
reduction in time in hypoglycaemia overnight and 46% overall in adults and adolescents1
Omnipod® 5 improved users’quality of life in clinical studies3,4
- Parents of children using Omnipod 5 reported improvements in quality of life, sleep quality, confidence avoiding hypoglycemia, and mental well-being3,4
- Adults using Omnipod reported improvements in diabetes distress, stress eating, confidence avoiding hypoglycemia, and mental wellbeing3,4
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs [Adults N=128, aged 14-70 years, Children N=112, aged 6-13.9 years). Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.89% vs. 1.32%, P<0.0001; 2.21% vs. 1.78, P=0.8153, respectively. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents and children, respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, P<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), P<0.0001). Average time above range (>10 mmol/L or >180 mg/dL) in children: ST vs. 3-month Omnipod 5 = 45.3% vs. 30.2%. Change is relative comparison. Average time above range (>10 mmol/L or >180 mg/dL) in adults: ST vs Omnipod 5: 32.4% vs. 24.7%. Change is relative. Mean time in hypoglycaemic range (<3.9 mmol/L or <70mg/dL as measured by CGM; 12AM - < 6AM) in adults as measured by CGM: ST: 2.07% vs. Omnipod 5: 0.82%, P<0.0001 Comparison is a relative change.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.43% vs. 2.46%, P=0.0204. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in very young children (2 - 5.9 yrs) as measured by CGM: ST = 57.2%, 3-mo Omnipod 5 = 68.1%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use: 7.4% vs 6.9% or 57 mmol/mol vs 53 mmol/mol, P<0.05.
3. Hood KK et al. Diabetes Obes Metab. 2024. Study of 81 children aged 6-11.9 with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean caregiver self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.34 vs 3.59, p-value <0.0001, respectively. During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively. During the Omnipod 5 pivotal trial, adults aged 18-70 (N=115) experienced an improved diabetes distress survey score after 3 months of AID use: mean: 1.48 vs. 1.64 (P < 0.001). During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively. During the Omnipod 5 pivotal trial, parents of children 6-11.9 years (N=82) experienced an improvement in sleep quality survey score after 3 months of Omnipod 5 use compared to standard therapy: mean PSQI Overall Sleep Quality Subscore = 0.70 vs 1.12, respectively.
4. Polonsky WH et al. Diabetes Res Clin Pract 2022;190:109998. Study of 115 adults aged 18-70 years with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.52 vs 3.65, p-value = 0.0002, respectively. Adults aged 18-70 (N=115) experienced reduction in eating distress score in T1-DDS survey after 3 months of Omnipod 5 use vs baseline: 1.73 vs. 1.97, (P=0.0003), respectively.
Initiating a Patient on Omnipod 5
Help your patients get a successful start on Omnipod® 5.
Omnipod 5 Simplifies Data Management
With Glooko®, your patient can access all of their diabetes information in one easy-to-use platform and easily share it with you.
Omnipod 5 Resources for your Patients
All of the resources, all in one place. Visit our resources page for User Guides, How-to-Videos, FAQs and more.
Important Safety Information: The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin. The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is indicated for use with NovoLog®/NovoRapid®, Humalog® /Liprolog ® ,Trurapi®/Truvelog®/Insulin aspart Sanofi®, Kirsty®, and Admelog/Insulin lispro Sanofi U-100 insulin.
Warning: SmartAdjust technology should not be used by anyone under the age of 2 years old or by people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.
Refer to the Omnipod® 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.